Cusabio Apoptosis Recombinants
Abstract
The process of programmed cell death, or apoptosis, is generally characterized by distinct morphological features and energy-dependent biochemical mechanisms. Apoptosis Recombinants is considered a vital component of several processes including normal cell turnover, proper development and function of the immune system, hormone-dependent atrophy, embryonic development, and chemical-induced cell death. Inappropriate apoptosis (either too little or too much) is a factor in many human conditions, including neurodegenerative diseases, ischemic damage, autoimmune disorders, and many types of cancer. The ability to modulate the life or death of a cell is recognized for its immense therapeutic potential.
Therefore, research continues to focus on the elucidation and analysis of the cell cycle machinery and signalling pathways that control cell cycle arrest and apoptosis. To that end, the field of apoptosis research has advanced at an alarmingly fast rate. Although many of the key apoptotic proteins have been identified, the molecular mechanisms of action or inaction of these proteins remain to be elucidated. The objective of this review is to provide an overview of current knowledge about the apoptosis process, including morphology, biochemistry, the role of apoptosis in health and disease, detection methods, as well as a discussion of possible ways apoptotic alternatives.
Keywords: Apoptosis, programmed cell death, intrinsic/extrinsic pathway, granzyme A/B, perforin, autophagy
Distinguish apoptosis from necrosis
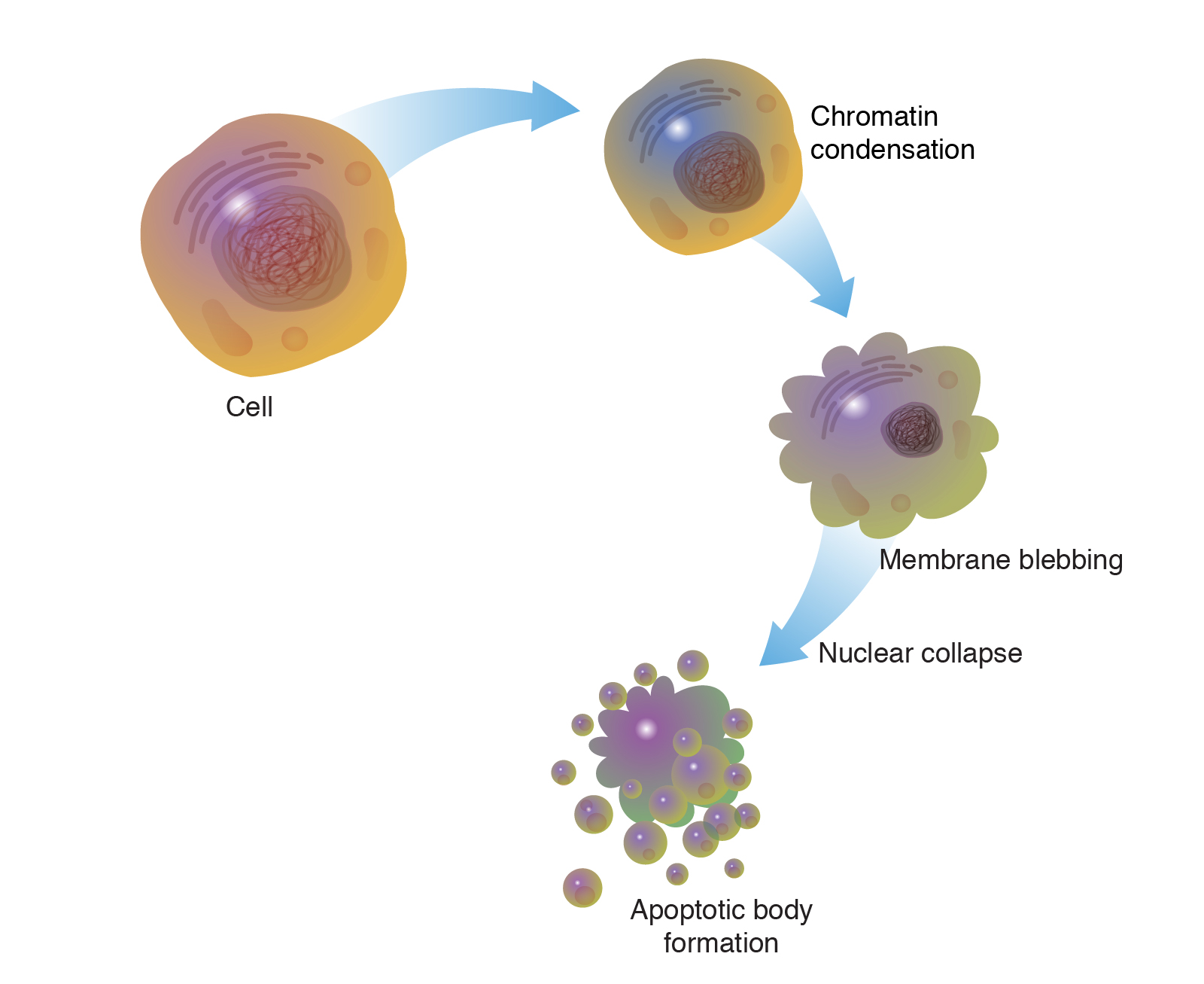
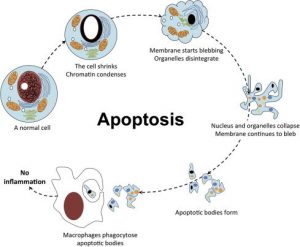
The alternative to apoptotic cell death is necrosis, which is considered a toxic process in which the cell is a passive victim and follows an energy-independent mode of death. But since necrosis refers to the degradative processes that occur after cell death, it is considered by some to be an inappropriate term to describe a mechanism of cell death. Therefore, oncosis is used to describe a process that leads to necrosis with karyolysis and cell swelling, while apoptosis leads to cell death with cell shrinkage, pyknosis, and karyorrhexis.

Therefore, the terms “oncotic cell death” and “oncotic necrosis” have been proposed as alternatives to describe cell death that is accompanied by cellular inflammation, but these terms are not widely used at this time (Majno and Joris, 1995; Levin et al., 1999). Although the mechanisms and morphologies of apoptosis and necrosis differ, these two processes overlap. Evidence indicates that necrosis and apoptosis represent morphological expressions of a shared biochemical network described as the “apoptosis-necrosis continuum” (Zeiss, 2003).
For example, two factors that will convert an ongoing apoptotic process into a necrotic process include a decrease in the availability of caspases and intracellular ATP (Leist et al., 1997; Denecker et al., 2001). Whether a cell dies by necrosis or apoptosis depends in part on the nature of the cell death signal, the type of tissue, the stage of tissue development, and the physiological milieu (Fiers et al., 1999; Zeiss, 2003).
Using conventional histology, it is not always easy to distinguish apoptosis from necrosis, and they can occur simultaneously depending on factors such as the intensity and duration of the stimulus, the degree of ATP depletion, and the availability of caspases (Zeiss, 2003). Necrosis is a passive and uncontrolled process that usually affects large fields of cells, while apoptosis is controlled and energy-dependent and can affect individual cells or groups of cells. Necrotic cell injury is mediated by two main mechanisms; interference with the cell’s energy supply and direct damage to cell membranes.
Physiological apoptosis
The role of apoptosis in normal physiology is as significant as that of its counterpart, mitosis. It demonstrates a complementary but opposite role to mitosis and cell proliferation in the regulation of various cell populations. It is estimated that to maintain homeostasis in the adult human body, about 10 billion cells are produced each day just to balance those that die by apoptosis (Renehan et al., 2001). And that number can increase significantly when apoptosis increases during normal development and ageing or during disease.
Apoptosis is critically important during various developmental processes. For example, both the nervous system and the immune system arise through the overproduction of cells. This initial overproduction is followed by the death of those cells that fail to establish functional synaptic connections or productive antigen specificities, respectively (Nijhawan et al., 2000; Opferman and Korsmeyer, 2003).
Apoptosis is also necessary to rid the body of cells invaded by pathogens and is a vital component of wound healing, as it is involved in the removal of inflammatory cells and the evolution of granulation tissue into scar tissue (Greenhalgh, 1998). . Dysregulation of apoptosis during wound healing can lead to pathological forms of scarring, such as excessive scarring and fibrosis. Apoptosis is also required to eliminate activated or autoaggressive immune cells either during maturation in central lymphoid organs (bone marrow and thymus) or in peripheral tissues (Osborne, 1996).
In addition, apoptosis is critical for remodellings in the adult, such as follicular atresia of the postovulatory follicle and postweaning mammary gland involution, to name a few examples (Tilly, 1991; Lund et al., 1996). Also, as organisms age, some cells begin to deteriorate at a faster rate and are eliminated by apoptosis. One theory is that oxidative stress plays a major role in the pathophysiology of age-induced apoptosis through accumulated free radical damage to mitochondrial DNA (Harman, 1992; Ozawa, 1995). It is clear that apoptosis has to be tightly regulated, as too little or too much cell death can lead to pathologies, including developmental defects, autoimmune diseases, neurodegeneration or cancer.
Apoptosis inhibition
There are many pathological conditions that exhibit excessive apoptosis (neurodegenerative diseases, AIDS, ischemia, etc.) and can therefore benefit from artificial inhibition of apoptosis. As our understanding of the field evolves, the identification and exploitation of new targets remain a considerable focus of attention (Nicholson, 2000). A shortlist of possible methods of anti-apoptotic therapy includes stimulation of the IAP (inhibitor of apoptosis) protein family, caspase inhibition, PARP (poly [ADP-ribose] polymerase) inhibition, stimulation of the PKB/Akt (protein kinase B) pathway and inhibition of Bcl-2 proteins.
The IAP family of proteins is perhaps the most important regulator of apoptosis due to the fact that it regulates both the intrinsic and extrinsic pathways (Deveraux and Reed, 1999). Eight human IAP proteins have now been identified, although XIAP (X-linked mammalian apoptosis inhibitory protein) and survivin remain the best-known members (Silke et al., 2002; Colnaghi et al., 2006). Until now, members of the IAP family have been investigated as therapeutic targets for the treatment of stroke, spinal cord injury, multiple sclerosis, and cancer.
The synthetic non-specific caspase inhibitor z-VAD-fmk was shown to reduce the severity of myocardial reperfusion injury in rat and mouse models of myocardial infarction (Mocanu et al., 2000). Specific inhibitors of caspase activity may also be of benefit. ICE (interleukin-1 beta converting enzyme), also called caspase I, is a cysteine protease that appears to mediate intracellular protein degradation during apoptosis (Livingston, 1997). ACE inhibitors have been developed to treat rheumatoid arthritis and other inflammatory conditions by reducing interleukin 1β (Le and Abbenante, 2005).
Due to the dual role of PARP-1 in both DNA repair and apoptosis, pharmacological use of PARP-1 inhibitors may attenuate ischemic and inflammatory cell and organ injury or may increase the cytotoxicity of antitumor agents ( Graziani and Szabo, 2005). Recent research with PARP-1 knockout mice indicates that the use of PARP-1 inhibitors may be an effective therapy for injury associated with myocardial ischemia and reperfusion injury (Zhou et al., 2006). Infusion of insulin-like growth factor 1 (IGF-1), which stimulates PKB/Akt signalling and promotes cell survival, was shown to be beneficial in animal models of myocardial ischemia (Fujio et al., 2000).
Other studies with transgenic models of cardiac ischemia and global cerebral ischemia indicate that inhibition of Bax expression and/or function can prevent cytochrome c release from mitochondria, inhibit mitochondrial membrane potential lowering, and protect cells against apoptosis (Hochhauser et al., 2003; Hetz et al., 2005). The potential therapeutic modalities mentioned here represent just a few of the past and current research efforts in this field. As the molecular and biochemical complexities of apoptosis are elucidated, new therapeutic strategies will continue to evolve.
Conclusions
Apoptosis is considered a carefully regulated energy-dependent process, characterized by specific morphological and biochemical features in which caspase activation plays a central role. Although many of the key apoptotic proteins that are activated or deactivated in apoptotic pathways have been identified, the molecular mechanisms of action or activation of these proteins are not fully understood and are the subject of continuing research.
The importance of understanding the mechanical machinery of apoptosis is vital because programmed cell death is a component of both health and disease, being initiated by various physiological and pathological stimuli. Furthermore, the widespread involvement of apoptosis in the pathophysiology of disease lends itself to therapeutic intervention at many different checkpoints. Understanding the mechanisms of apoptosis and other variants of programmed cell death at the molecular level provides deeper insights into various disease processes and thus may influence therapeutic strategy.